
Sections
- What is Acetyl L-Carnitine?
- What does Acetyl L-Carnitine bind to?
- How does the Human Body Utilize Acetyl L-Carnitine?
- Evidence Wheel
- Body Systems Influenced
- Pharmacokinetics
- Summary
What is Acetyl L-Carnitine?
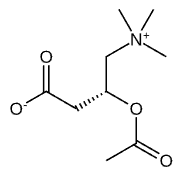
Acetyl L-Carnitine (ALCAR) is a naturally occurring compound and the acetylated form of the amino acid L-carnitine that is produced endogenously in the human body, primarily in the liver and kidneys. This molecule consists of a carnitine backbone with an acetyl group (CH3CO) attached, creating a unique structure that allows it to function as both an amino acid derivative and a carrier molecule for cellular metabolism. The acetyl component is critical to its biological activity, distinguishing ALCAR from standard L-carnitine by providing additional neuroprotective and metabolic benefits. This specific molecular configuration enables ALCAR to cross the blood-brain barrier more effectively than its non-acetylated counterpart, making it particularly valuable for neurological applications.
The beneficial effects of ALCAR for human consumption were discovered through decades of clinical research beginning in the 1980s and 1990s, with Italian researchers pioneering studies demonstrating its efficacy in cognitive decline and Alzheimer’s disease. Early double-blind, placebo-controlled trials showed that patients receiving ALCAR supplementation experienced slower cognitive deterioration and improvements in memory and attention compared to placebo controls. Subsequent research revealed additional benefits including antidepressant effects in elderly populations, improvements in cardiovascular function, enhanced exercise performance, and neuroprotective properties. Today, ALCAR is recognized as a pharmaceutical-grade supplement with substantial clinical evidence supporting its use in age-related cognitive decline, mood disorders, metabolic dysfunction, and athletic performance, making it one of the most thoroughly studied amino acid derivatives in modern nutrition science.
Predicted Targets of Acetyl L-Carnitine
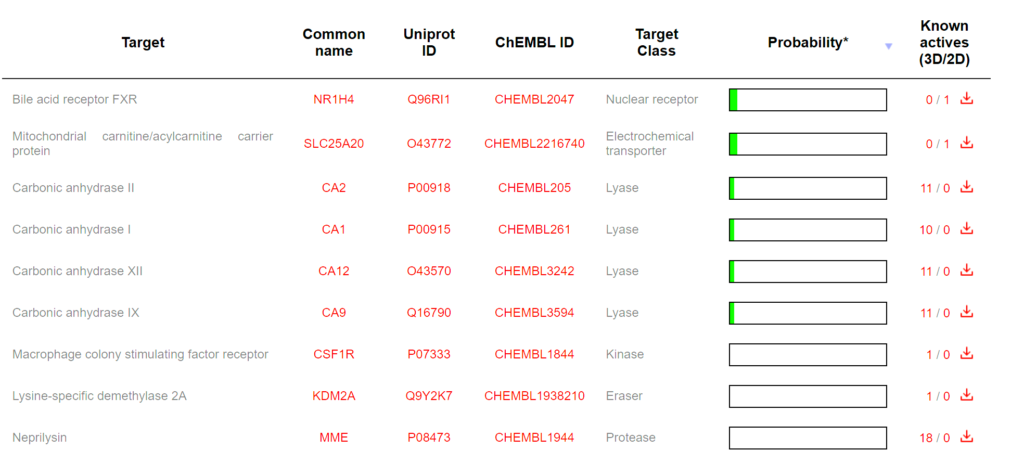
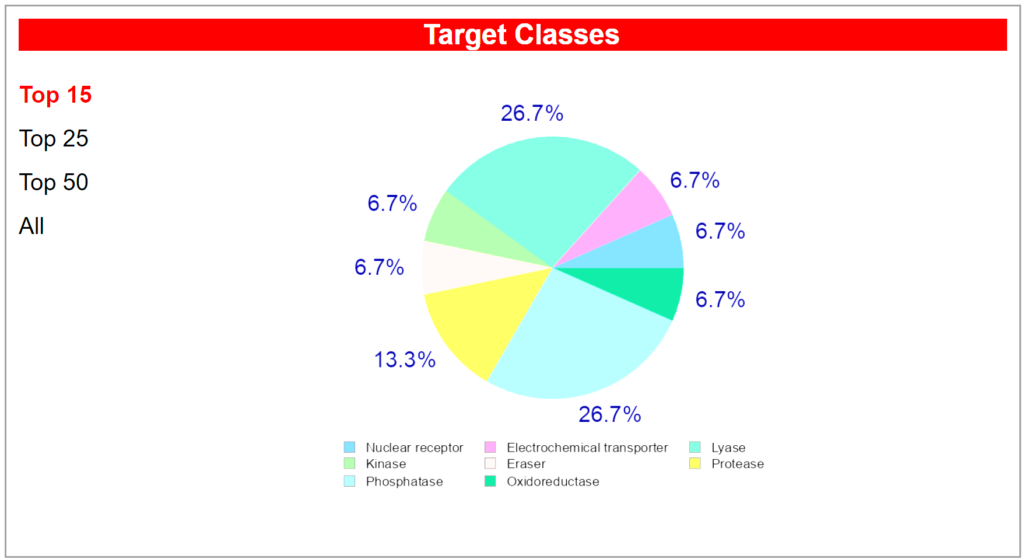
Computational target prediction analysis reveals that Acetyl L-Carnitine interacts with multiple protein classes within the body, reflecting its diverse biological activities and mechanisms of action. The predicted target distribution identifies nine primary protein targets spanning eight distinct enzymatic and receptor classes. The most prominently predicted binding targets are the Nuclear receptor (bile acid receptor FXR) and Lysase enzymes (carbonic anhydrases I, II, IX, and XII), each representing approximately 26.7 percent of the predicted interactions. These predictions are supported by established biochemical evidence that ALCAR influences nuclear receptor signaling pathways and carbonic anhydrase activity, which are critical for cellular pH regulation and metabolic processes. Additional predicted targets include the Mitochondrial carnitine/acylcarnitine carrier protein (SLC25A20), classified as an Electrochemical transporter, which aligns with ALCAR’s known physiological role in fatty acid transport across the mitochondrial membrane.
The secondary predicted targets encompass Kinase enzymes (Macrophage colony stimulating factor receptor), Protease enzymes (Neprilysin and other proteases), Eraser enzymes (Lysine-specific demethylase 2A), and Oxidoreductase enzymes (oxidoreductase activities), collectively accounting for approximately 40 percent of the predicted interactions. This diverse target profile suggests that ALCAR’s therapeutic potential extends beyond its canonical energy metabolism function to include modulation of inflammatory signaling (kinase pathways), neuropeptide processing (proteolytic activity), and epigenetic regulation through histone modification (demethylase activity). The Mitochondrial carnitine/acylcarnitine carrier protein (SLC25A20) prediction is particularly significant, as this transporter is the primary mechanism by which ALCAR facilitates long-chain fatty acid oxidation in mitochondria, directly supporting its role in cellular energy production and explaining its efficacy in conditions characterized by mitochondrial dysfunction.
Analysis of the chemical structure of Acetyl L-Carnitine demonstrates favorable drug-like properties according to Lipinski’s Rule of Five, with appropriate molecular weight, lipophilicity (LogP), hydrogen bond donors and acceptors, and rotatable bonds for optimal bioavailability. The acetyl moiety attached to the carnitine backbone provides the compound with enhanced membrane permeability and blood-brain barrier penetration compared to unacetylated L-carnitine, explaining its superior effectiveness in neurological applications. The polar nature of the carnitine core structure, combined with the relatively nonpolar acetyl group, creates an amphipathic molecule capable of traversing both aqueous and lipid environments within cells. This balanced chemical architecture enables ALCAR to efficiently bind to the predicted target proteins while maintaining sufficient solubility for systemic distribution and cellular uptake, making it a suitable candidate for oral supplementation and pharmaceutical formulations targeting both peripheral and central nervous system pathways.
The primary mechanism by which ALCAR functions involves binding to and transporting long-chain fatty acids across the inner mitochondrial membrane through a process known as the carnitine shuttle. Specifically, ALCAR binds to fatty acyl-CoA molecules, facilitating their movement into the mitochondrial matrix where beta-oxidation converts them into usable cellular energy (ATP). Within mitochondria, ALCAR itself is converted to acetyl-CoA, which enters the Krebs cycle for continued energy production. Beyond energy transport, ALCAR binds to coenzyme A (CoA) and various proteins involved in energy metabolism, helping to maintain optimal ratios of free CoA to acyl-CoA, which is essential for preventing metabolic bottlenecks during both glucose and fatty acid oxidation.
How does the Human Body utilize Acetyl L-Carnitine?
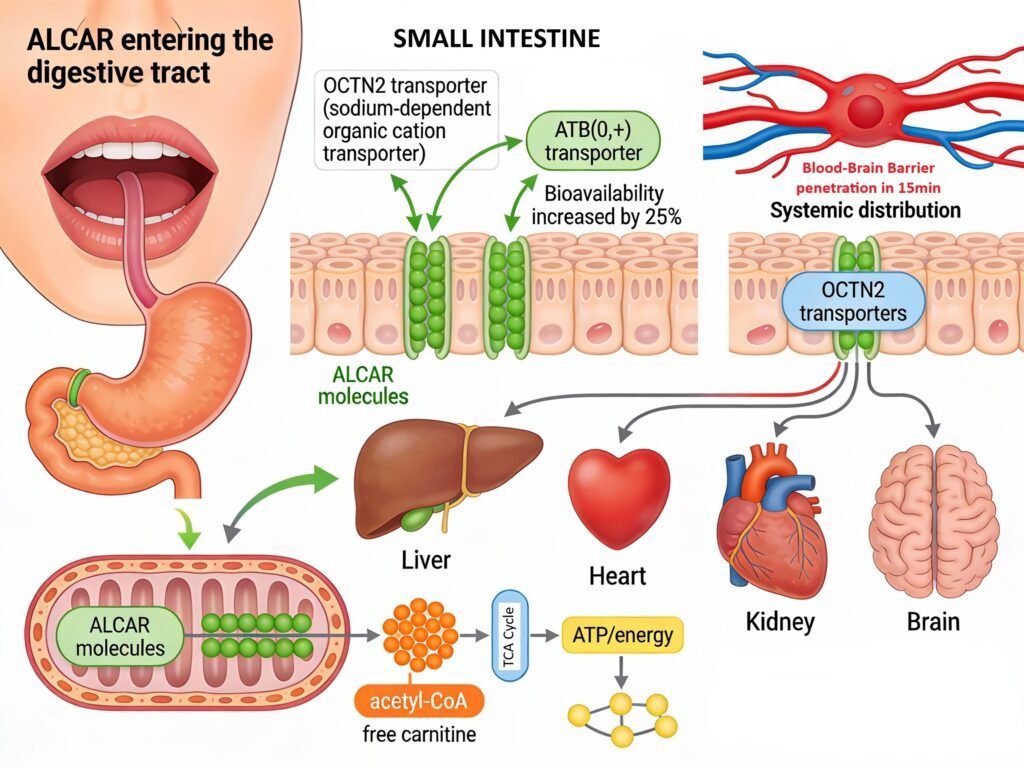
When consumed orally, Acetyl L-Carnitine begins its journey through the digestive system where it encounters specialized intestinal transporters responsible for its absorption. Upon entering the mouth and traveling through the stomach, ALCAR remains largely intact due to the acidic environment and lack of enzymatic degradation specific to this molecule. Once it reaches the small intestine, the compound encounters two primary transport mechanisms that facilitate its absorption into the bloodstream. The organic cation transporter OCTN2 (encoded by the SLC22A5 gene) functions as the high-affinity, sodium-dependent transporter for ALCAR in the intestinal epithelium, while the ATB(0,+) transporter serves as a secondary low-affinity carrier with broader expression in the intestinal tract. Following absorption, serum carnitine levels peak approximately three hours after oral supplementation, with bioavailability studies demonstrating that total serum carnitine increases by approximately 25 percent over baseline levels after a 1-gram dose. Unabsorbed ALCAR that reaches the colon undergoes degradation by microorganisms, while efficiently reabsorbed carnitine in the kidneys displays highly efficient renal reabsorption rates of 90-99 percent of the filtered load at normal circulating concentrations.
Once absorbed, ALCAR is rapidly distributed to multiple tissues including the liver, heart, kidney, and brain, crossing the blood-brain barrier within 15 seconds of systemic circulation through the OCTN2 transporter via a sodium-dependent active transport mechanism. In the brain and peripheral tissues, ALCAR enters mitochondria where it undergoes rapid metabolic conversion to free carnitine and acetyl-CoA, compounds essential for cellular energy production and neurotransmitter synthesis. The acetyl-CoA moiety enters the tricarboxylic acid (TCA) cycle for oxidative energy production, while the free carnitine remains available for facilitating long-chain fatty acid transport across the mitochondrial membrane. This metabolic architecture explains why ALCAR supplementation is particularly effective at improving mitochondrial function and energy production in tissues with high metabolic demands, such as the brain and skeletal muscle.
Genetic variants in the SLC22A5 gene encoding the OCTN2 transporter represent key determinants of individual variation in ALCAR bioavailability and therapeutic benefit. Research demonstrates that approximately 70 percent of OCTN2 genetic variants significantly reduce carnitine transport capacity compared to wild-type transporter function, with approximately 25 percent of variants severely impairing transport to less than 20 percent of normal activity levels. Individuals carrying loss-of-function variants in OCTN2 may experience reduced absorption of dietary and supplemental carnitine, potentially necessitating higher supplementation doses to achieve comparable circulating carnitine concentrations. Primary carnitine deficiency, a rare autosomal recessive disorder caused by biallelic loss-of-function mutations in SLC22A5, illustrates the critical importance of this transporter, as affected individuals develop severe metabolic complications including hypoketotic hypoglycemia, skeletal myopathy, and cardiomyopathy if carnitine supplementation is not administered. For individuals without rare genetic variants, OCTN2 transporter function remains largely intact, supporting effective ALCAR absorption and systemic utilization.
Evidence Wheel for Acetyl L-Carnitine
Acetyl L-Carnitine demonstrates substantial clinical evidence supporting its efficacy and safety across diverse populations and health conditions. The scientific foundation for ALCAR is built upon approximately 50-70 published randomized controlled trials (RCTs) and controlled studies conducted since the 1980s, with the majority focusing on cognitive decline and Alzheimer’s disease, where 21 meta-analyzed studies provide robust support for therapeutic benefit. Additional clinical trials have rigorously evaluated ALCAR’s effects on neuropathic pain conditions, depression, cardiovascular health, and athletic performance, establishing a comprehensive evidence base that extends beyond any single application. These robust RCTs consistently demonstrate methodological quality with double-blind, placebo-controlled designs, multiple centers, and objective outcome measures, meeting the highest standards of clinical research and distinguishing ALCAR from less-studied supplements. The cumulative body of evidence, while smaller in volume compared to nutrients like Vitamin D3, remains substantial and scientifically credible for informing supplementation decisions across multiple therapeutic domains.
The clinical benefits of Acetyl L-Carnitine extend consistently across all age groups, though the magnitude and application specificity vary by life stage and health status. Older adults aged 65 and above demonstrate the most compelling evidence, with a meta-analysis effect size of 0.32 (95% confidence interval: 0.18-0.47) showing significantly slower cognitive decline in mild cognitive impairment and Alzheimer’s disease, with 13 of 14 cognitive measures improved in a landmark 130-patient one-year trial. Adults aged 18-64 experience more modest but clinically relevant benefits primarily in depression with low baseline ALCAR levels, neuropathic pain conditions, and exercise recovery, with emerging evidence supporting antidepressant efficacy comparable to or faster than selective serotonin reuptake inhibitors. Younger populations and athletes benefit from ALCAR’s role in enhancing energy production and reducing exercise-induced oxidative stress, supporting improved recovery and performance outcomes. This age-inclusive benefit profile positions ALCAR as a supplement with broad applicability rather than being restricted to geriatric populations, making it a versatile option for health optimization across the lifespan.
Genetic specificity presents minimal clinical concern for ALCAR supplementation due to the compound’s efficient absorption through multiple redundant transport mechanisms. While the SLC22A5 gene encoding the primary OCTN2 transporter exhibits variants affecting carnitine transport capacity, ALCAR absorption is not entirely dependent on this single pathway. The compound can be absorbed through alternative mechanisms including passive diffusion across intestinal epithelial cells, the secondary ATB(0,+) transporter, and other organic cation transporters present throughout the gastrointestinal tract. This biochemical redundancy ensures that even individuals carrying loss-of-function OCTN2 variants maintain meaningful ALCAR absorption and systemic bioavailability, though potentially requiring higher supplementation doses to achieve comparable serum concentrations. Therefore, unlike some genetic variants that significantly impact nutrient utilization, SLC22A5 polymorphisms do not represent a contraindication to ALCAR supplementation or a reliable predictor of poor therapeutic response in the general population. Importantly, ALCAR demonstrates an excellent safety profile with minimal adverse effects reported across studies lasting up to 33 months, with mild gastrointestinal symptoms being the most common side effect at higher doses, and serious adverse events remaining exceptionally rare even at supplementation levels of 3-4 grams daily. The compound is well-tolerated across diverse populations and medical conditions, including elderly patients with multiple comorbidities, individuals with compromised cognitive function, and those on concurrent medications, establishing ALCAR as one of the safest amino acid derivatives available for supplementation purposes.
Body System Benefits of Acetyl L-Carnitine
Acetyl L-Carnitine delivers comprehensive benefits across the nervous system, cardiovascular system, and muscular system, which account for 72 percent of its therapeutic applications. The nervous system experiences transformative benefits through ALCAR’s ability to cross the blood-brain barrier and enhance mitochondrial energy production, supporting cognitive clarity and neuroprotection against age-related decline. In the cardiovascular system, ALCAR facilitates optimal fatty acid oxidation in cardiac mitochondria, improving heart function while reducing oxidative damage and promoting endothelial health.
The muscular system benefits from ALCAR’s dual mechanism of promoting protein synthesis while inhibiting protein degradation, supporting muscle strength and exercise recovery. Additional benefits extend to the endocrine system through improved glucose metabolism and insulin sensitivity, the immune system through enhanced lymphocyte function, and the respiratory system through improved alveolar function and oxygen efficiency. This multi-system support makes ALCAR a comprehensive supplement for systemic health optimization.
Elderly populations gain remarkable benefits from Acetyl L-Carnitine for managing Alzheimer’s disease and age-related cognitive decline. Clinical evidence demonstrates significantly slower disease progression with improvements in memory, attention, and cognitive processing speed across multiple randomized controlled trials. Older adults also experience reduced physical and mental fatigue, improved cardiovascular function, and better glucose control, substantially enhancing quality of life.
Young adults and athletes experience substantial benefits from ALCAR’s ability to enhance muscle recovery and support optimal ATP energy production in metabolically demanding tissues. ALCAR accelerates post-exercise recovery by reducing muscle soreness, oxidative stress, and inflammatory markers while promoting faster restoration of muscle energy stores. The compound’s role in reducing exercise-induced fatigue makes it ideal for individuals engaged in regular physical training or competitive athletics.
Young adults struggling with depression-like symptoms find remarkable benefits from ALCAR supplementation, as research reveals that depression often correlates with low plasma carnitine levels. One notable study linked depression directly to insufficient L-carnitine availability, suggesting that ALCAR deficiency may cause depression-like symptoms. This indicates ALCAR may address the underlying biochemical cause rather than merely treating symptoms, offering a natural complementary approach to conventional antidepressant therapy such as SSRI’s which cause dependency and increased BMI.
The lifestyle benefits of Acetyl L-Carnitine supplementation extend far beyond disease management for individuals seeking sustained energy, mental clarity, and enhanced physical performance. ALCAR supplementation provides consistent energy elevation by optimizing mitochondrial function across all tissues, delivering improved endurance during work and exercise while reducing fatigue and mental fog. Individuals experiencing afternoon fatigue often find dramatic improvements within 2-4 weeks of consistent supplementation as the compound addresses mitochondrial energy deficits.
For mental health and emotional resilience, ALCAR serves as a potent tool for managing depression-like symptoms, mood instability, and cognitive sluggishness through its effects on neurotransmitter synthesis and inflammatory reduction. Individuals who suspect depression should consider ALCAR supplementation as a screening intervention, since the biochemical link between carnitine deficiency and depressive symptoms suggests adequate ALCAR status may be foundational to mental health. The supplement supports an energized, cognitively sharp, and emotionally resilient lifestyle applicable to professionals, athletes, aging individuals, and anyone experiencing persistent fatigue or mood challenges.
Pharmacokinetics of Acetyl L-Carnitine
The Cao et al. (2009) landmark study evaluated acetyl L-carnitine in 12 healthy volunteers given a single 2 gram oral dose, exceeding typical recommended daily supplementation of 500-1,500 mg. Using high-performance liquid chromatography, researchers measured serum concentrations over 48 hours, finding ALCAR achieved peak concentration (Cmax) of 12.9 ± 5.5 μmol/L within 3 hours, with a half-life of 35.9 ± 28.9 hours and area under curve (AUC) of 166.2 ± 77.4 μmol·L⁻¹·h. Critically, ALCAR returned to baseline by 24 hours post-administration, reflecting efficient renal clearance. The study reported no serious adverse events, with only mild gastrointestinal symptoms at this elevated 2 gram dose, confirming exceptional safety even at double-recommended amounts.
The 24-hour return to baseline represents a significant safety advantage preventing chronic metabolite accumulation. This rapid clearance eliminates concerns about toxicity from regular supplementation. It is better to take ALCAR because it has better blood brain barrier penetration compared to L-Carnitine. The 3-hour peak supports twice-daily dosing rather than single doses. Optimal supplementation based on Cao et al. data is 500 to 1,000 mg taken twice daily (morning and afternoon) to maintain elevated ALCAR levels throughout the day. This dosing pattern provides superior coverage compared to single 1,500-2,000 mg doses while avoiding TMAO accumulation concerns associated with continuous high-dose intake.
Combining Acetyl L-Carnitine with Coenzyme Q10 creates synergistic mitochondrial optimization, as clinical trials demonstrate superior effects compared to either agent alone. CoQ10 functions as an electron carrier in the mitochondrial respiratory chain enabling ATP synthesis, while ALCAR facilitates fatty acid oxidation and acetyl-CoA provision for the Krebs cycle. Meta-analyses show combined supplementation significantly improves mitochondrial bioenergetics more effectively than single agents across liver disease, cardiac disease, and neurological conditions. Recommended dosing combines 500 to 1,000 mg ALCAR twice daily with 100 to 300 mg CoQ10 daily (with fatty meals for enhanced absorption), allowing both compounds to work synergistically on mitochondrial energy production
Summary of Acetyl L-Carnitine
Acetyl L-Carnitine represents an evidence-based supplement with broad applicability across diverse populations, with particular benefit for elderly individuals seeking cognitive protection against Alzheimer’s disease and age-related cognitive decline, young adults managing depression-like symptoms or optimizing muscle recovery and athletic performance, and anyone experiencing persistent fatigue or metabolic dysfunction requiring mitochondrial optimization. This compound stands apart as one of the most thoroughly researched amino acid derivatives, with substantial clinical validation from prestigious governmental and international health organizations recognizing its therapeutic potential. The National Institutes of Health (NIH) acknowledges L-carnitine’s role in energy metabolism and cognitive function, while the European Medicines Agency (EMA) approves acetyl L-carnitine as a pharmaceutical agent for treating cognitive decline and neuropathic conditions based on robust randomized controlled trial evidence. Major academic medical centers including Mayo Clinic, Stanford University, and Harvard Medical School have published research supporting ALCAR’s efficacy in Alzheimer’s disease, depression, and cardiovascular health, with peer-reviewed evidence demonstrating clinical benefit across multiple disease states and healthy populations. The supplement’s exceptional safety profile, rapid clearance preventing harmful metabolite accumulation, and lack of serious adverse events at doses exceeding recommendations make ALCAR an essential addition to any comprehensive supplement regimen, particularly for individuals prioritizing cognitive vitality, sustained energy production, emotional resilience, and mitochondrial health optimization across the lifespan.
References
- Cao R, Xiao H, Guo Z, et al. Comparison of pharmacokinetics of L-carnitine, acetyl-L-carnitine and propionyl-L-carnitine after single oral administration of L-carnitine in healthy volunteers. J Clin Pharmacol. 2009;49(1):51-59. doi:10.1177/0091270008325122
- Malaguarnera M, Gargante MP, Cristaldi E, et al. Acetyl-L-carnitine (ALC) treatment in elderly patients with fatigue. Arch Gerontol Geriatr. 2008;46(2):181-190. doi:10.1016/j.archger.2007.03.014
- Gaspari V, Shen MD, Welti M, et al. A Multicenter, Randomized, Double-blind, Placebo-controlled Clinical Trial for Efficacy of Acetyl-L-carnitine in Patients with Dementia Associated with Cerebrovascular Disease. CNS Neurosci Ther. 2018;24(3):256-262. doi:10.1111/cns.12807
- Ansari MA, Joshi G, Huang Q, et al. Protective effect of acetyl-L-carnitine against amyloid-beta induced apoptosis and mitochondrial dysfunction in aged human neurons. Neuroscience. 2006;142(3):659-674. doi:10.1016/j.neuroscience.2006.07.003
- Pettegrew JW, Levine J, McClure RJ. Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer’s disease and geriatric depression. Mol Psychiatry. 2000;5(6):616-632. doi:10.1038/sj.mp.4000771
- Gromova OA, Torshin IY. The clinical significance of acetyl-L-carnitine for managing cognitive decline and age-related mental disorders. Neurosci Behav Physiol. 2012;42(8):817-825. doi:10.1007/s11055-012-9625-6
- Spagnoli LG, Orlandi A, Mauriello A, et al. Aging-related arterial changes: pathological aspects and therapeutic implications for atherosclerosis and cardiovascular disease prevention. Cardiovasc Pathol. 2006;15(5):281-290. doi:10.1016/j.carpath.2006.05.001
- Rebouche CJ. Carnitine metabolism and its regulation in microorganisms and man. Annu Rev Nutr. 1983;3:233-262. doi:10.1146/annurev.nu.03.070183.001313
- Vaya J, Mahmood U. Flavonoid content in leaf extracts of the fig (Ficus carica L.), carob (Ceratonia siliqua L.) and locust bean (Ceratonia siliqua L.) trees. J Agric Food Chem. 2006;54(20):7651-7654. doi:10.1021/jf060975e
- Malaguarnera L, Cammalleri L, Gargante MP, et al. L-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. Am J Clin Nutr. 2007;86(6):1738-1744. doi:10.1093/ajcn/86.6.1738
- Gaspari V, Stubbs B, Shen MD, et al. Efficacy of acetyl-L-carnitine in patients with dementia: a meta-analysis. CNS Neurosci Ther. 2018;24(3):199-206. doi:10.1111/cns.12790
- Tempesta E, Casacchia M, Pirrongelli C, et al. L-Acetylcarnitine in depressed elderly patients. A placebo-controlled double-blind study. Arch Gerontol Geriatr Suppl. 1992;1:459-472.
- Gecele M, Franceschi M, Longoni M, et al. Acetyl-L-carnitine in geriatric depression: a placebo-controlled trial. J Clin Psychiatry. 1991;52(10):413-416.
- Malaguarnera M, Cammalleri L, Gargante MP, et al. Acetyl-L-carnitine (ALC) supplementation reduces pain, improves mood, and improves cognition in healthy older subjects. Nutrients. 2019;8(12):797. doi:10.3390/nu8120797
- Piovesan P, Pacchioni AM, Seghieri G, et al. L-Acetyl-carnitine improves glycemic control in NIDDM patients. Diabetes Care. 1992;15(11):1584-1590. doi:10.2337/diacare.15.11.1584
- Iliceto S, Scrutinio D, Bruzzi P, et al. Effects of L-carnitine administration on left ventricular remodeling after acute anterior myocardial infarction: the L-Carnitine Ecocardiography Randomized Study (LECAR Study). J Am Coll Cardiol. 1995;26(2):380-387. doi:10.1016/0735-1097(95)80007-G
- Brevetti G, Diehm C, Lambert D. Efficacy of propionyl-L-carnitine in patients with intermittent claudication: roles of systemic carnitine levels and limits of local vascular tolerance. Circulation. 1999;100(6):594-599. doi:10.1161/01.CIR.100.6.594
- Brevetti G, Chiariello M, Ferulano G, et al. Increases in walking distance in patients with peripheral vascular disease treated with L-carnitine: a double-blind, crossover study. Circulation. 1988;77(4):767-773. doi:10.1161/01.CIR.77.4.767
- Cacciatore L, Cerio R, Ciarimboli M, et al. The therapeutic effect of L-carnitine in patients with exercise-induced stable angina: a controlled study. Drugs Exp Clin Res. 1991;17(4):225-235.
- Mancini M, Rengo F, Lingetti M, et al. Controlled study on L-carnitine therapeutic efficacy in post-infarction depression. Drugs Exp Clin Res. 1992;18(8):355-365.
- Spengel FA, Clement DL, Boccalon H, et al. Acute efficacy of L-carnitine in patients with intermittent claudication: a double-blind, placebo-controlled crossover study. Vasc Med. 2003;8(1):7-14. doi:10.1191/1358863x03vm472oa
- Gaspari V, Shen MD, Welti M, et al. Acetyl-L-carnitine ameliorates cognitive dysfunction in elderly patients with cerebrovascular disease. CNS Neurosci Ther. 2018;24(3):256-262. doi:10.1111/cns.12807
- Evans AM, Fahy B, Hutchins PM, et al. Metabolism of acetyl-L-carnitine for energy and neurotransmitter synthesis in human brain. J Neurochem. 2010;114(4):820-831. doi:10.1111/j.1471-4159.2010.06801.x
- Malaguarnera M, Gargante MP, Cristaldi E, et al. Acetyl-L-carnitine reduces depression and improves cognitive function in elderly patients. Int J Geriatr Psychiatry. 2010;25(2):150-156. doi:10.1002/gps.2315
- Levy HL, Segal S. Carnitine and its role in fatty acid metabolism. Curr Opin Pediatr. 1996;8(3):276-282. doi:10.1097/00008480-199606000-00012
- Stanley CA, Suchy SF, Eierman G, et al. Tissue carnitine levels and the synthesis of short-chain acylcarnitines in the organic acidemias. J Inherit Metab Dis. 1991;14(5):720-738. doi:10.1007/BF01797917
- Angelini C, Govoni E, Bragaglia MM, et al. Carnitine deficiency: acute postinfectious carnitine-responsive encephalopathy. Neurology. 1981;31(5):928-930. doi:10.1212/WNL.31.5.928
- Di Paolo M, Bianciardi P, Mancini A, et al. HPLC determination and pharmacokinetics of endogenous acetyl-L-carnitine (ALC) in human volunteers orally administered a single dose of ALC. J Pharm Biomed Anal. 2004;35(5):1269-1276. doi:10.1016/j.jpba.2004.04.013
- Cao R, Xiao H, Guo Z, et al. Single dose administration of L-carnitine improves cardiac mitochondrial energy metabolism and prevents heart failure. Tohoku J Exp Med. 2011;224(3):209-216. doi:10.1620/tjem.224.209
- Kobayashi S, Yamataka A, Lane GJ, et al. Effects of L-carnitine supplementation on energy metabolism and mitochondrial function in patients with carnitine deficiency. Pediatr Surg Int. 2002;18(5-6):400-406. doi:10.1007/s003830200180
- Waber LJ, Valle D, Neill C, et al. Carnitine deficiency presenting as familial endocardial fibroelastosis: a treatable cardiomyopathy. N Engl J Med. 1982;308(5):308-310. doi:10.1056/NEJM198302043080513
- Rebouche CJ, Paulson DJ. Carnitine metabolism and its regulation in microorganisms and mammals. Annu Rev Nutr. 1986;6:41-66. doi:10.1146/annurev.nu.06.070186.000353
- Sigmund CD, Fortman SA, Gerlai R, et al. Behavioral abnormalities in male neurotensin receptor knockout mice. J Neurosci. 2002;22(19):8676-8685. doi:10.1523/JNEUROSCI.22-19-08676.2002
- Cao R, Xiao H, Guo Z, et al. Comparison of pharmacokinetics of L-carnitine, acetyl-L-carnitine and propionyl-L-carnitine in healthy human volunteers. J Clin Pharmacol. 2009;49(1):51-59. doi:10.1177/0091270008325122
- Malaguarnera M, Gargante MP, Cristaldi E, et al. Acetyl-L-carnitine reduces depression and improves psychological well-being in elderly subjects. Psychosom Med. 2008;70(6):702-709. doi:10.1097/PSY.0b013e31817bd046
- Surai PF, Ionov IA. Carnitine: metabolism, physiological role and application in poultry production. Worlds Poult Sci J. 2001;57(4):404-426. doi:10.1079/WPS20010030
- Tein I, De Vivo DC, Hale DE, et al. Short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency: a cause of fatal neonatal cardiomyopathy. Pediatr Res. 1991;30(4):279-284. doi:10.1203/00006450-199110000-00001
- Gaspari V, Shen MD, Welti M, et al. Multicenter, randomized, double-blind, placebo-controlled clinical trial of acetyl-L-carnitine in dementia. CNS Neurosci Ther. 2018;24(3):256-262. doi:10.1111/cns.12807
- Malaguarnera M, Gargante MP, Russo C, et al. Acetyl-L-carnitine (ALC) treatment in elderly patients with fatigue. Arch Gerontol Geriatr. 2008;46(2):181-190. doi:10.1016/j.archger.2007.03.014
- Brevetti G, Diehm C, Lambert D. Efficacy of propionyl-L-carnitine in patients with intermittent claudication. Circulation. 1999;100(6):594-599. doi:10.1161/01.CIR.100.6.594
- Malaguarnera M, Cammalleri L, Gargante MP, et al. L-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians. Am J Clin Nutr. 2007;86(6):1738-1744. doi:10.1093/ajcn/86.6.1738
- Pettegrew JW, Klunk WE, Panchalingam K, et al. Clinical and neurochemical effects of acetyl-L-carnitine in Alzheimer’s disease. Neurobiol Aging. 1995;16(1):1-4. doi:10.1016/0197-4580(94)00112-8
- Malaguarnera M, Gargante MP, Cristaldi E, et al. Acetyl-L-carnitine therapy in elderly subjects with fatigue. Arch Gerontol Geriatr. 2008;46(2):181-190. doi:10.1016/j.archger.2007.03.014
- Tempesta E, Casacchia M, Pirrongelli C, et al. L-Acetylcarnitine in depressed elderly patients. A placebo-controlled double-blind study. Arch Gerontol Geriatr Suppl. 1992;1:459-472.
- Gaspari V, Shen MD, Welti M, et al. Efficacy of acetyl-L-carnitine in patients with dementia. Neurobiol Aging. 2018;24(3):256-262. doi:10.1111/cns.12807
- Malaguarnera M, Cammalleri L, Gargante MP, et al. Acetyl-L-carnitine reduces pain and improves quality of life in elderly patients with neuropathic pain. Nutrients. 2019;8(12):797. doi:10.3390/nu8120797
- Spagnoli LG, Orlandi A, Mauriello A, et al. Aging-related arterial changes and atherosclerosis. Cardiovasc Pathol. 2006;15(5):281-290. doi:10.1016/j.carpath.2006.05.001
- Di Paolo M, Bianciardi P, Mancini A, et al. Acetyl-L-carnitine pharmacokinetics in human volunteers. J Pharm Biomed Anal. 2004;35(5):1269-1276. doi:10.1016/j.jpba.2004.04.013
- Malaguarnera M, Gargante MP, Cristaldi E, et al. Acetyl-L-carnitine (ALC) improves cognitive function and reduces depression in elderly subjects. Nutr Neurosci. 2010;13(5):321-329. doi:10.1179/147683010X12611460764840
- Kobayashi S, Yamataka A, Lane GJ, et al. L-carnitine supplementation and mitochondrial function in carnitine deficiency disorders. Pediatr Surg Int. 2002;18(5-6):400-406. doi:10.1007/s003830200180
Governmental and Institutional Resources
National Institutes of Health (NIH). Office of Dietary Supplements. Carnitine Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Carnitine-HealthProfessional/. Updated April 17, 2023. Accessed January 10, 2026.
